A luxurious metallic finish can quickly turn into a peeling nightmare when exposed to high-pH products, damaging your brand’s premium image. You must understand the chemical vulnerabilities of metallized glass to prevent shelf failures.
Yes, alkaline substances can severely corrode "electroplated" (vacuum metallized) glass bottles. High pH liquids penetrate the protective topcoat, reacting with the delicate metal layer—typically aluminum—causing rapid blackening, oxidation, and catastrophic delamination.

Can Alkaline Substances Corrode Electroplated Coatings on Glass Bottles?
The Illusion of "Electroplating"
In my two decades at FuSenglass, I have seen countless buyers confuse "electroplating" with the actual industrial process used on glass, which is usually Vacuum Metallization (PVM). True galvanic electroplating 1 requires a conductive surface, which glass is not. To achieve that mirror-like metallic shine, we typically apply a UV base coat, vaporize a thin layer of aluminum in a vacuum chamber, and seal it with a UV topcoat.
This distinction is critical because Aluminum—the core of 90% of "electroplated" bottles—is amphoteric 2. This means it dissolves in both acids and alkalis. If the alkaline substance breaches the protective topcoat, it eats the aluminum alive. The shiny silver turns into a dull grey or transparent patch, and hydrogen gas is released, often causing the coating to bubble and peel off.
The Mechanism of Alkali Attack
The corrosion process is twofold. First, the alkaline substance (like a heavy-duty cleanser or a high-pH hair removal cream) attacks the polymer topcoat. If this topcoat is a standard acrylic, it may undergo hydrolysis 3 (saponification) in high pH environments, softening and swelling.
Once the topcoat is compromised—even via a microscopic pinhole—the alkali reaches the metal layer. For aluminum, the reaction with Sodium Hydroxide (NaOH) is vigorous. It forms sodium aluminate 4 and hydrogen gas. The pressure from the gas blows the coating off the glass. This is why "electroplated" bottles are notoriously risky for alkaline products unless specifically engineered with high-resistance topcoats.
Corrosion Risk Overview by Layer
| Layer Component | Function | Reaction to Alkali (High pH) | Consequence |
|---|---|---|---|
| Topcoat (Lacquer) | Protects metal from air/touch. | Softens, swells, or hydrolyzes. | Barrier breach; loss of gloss. |
| Metal Layer (Al) | Provides the "Chrome" look. | Reacts to form Aluminate + $H_2$. | Blackening, transparency, peeling. |
| Metal Layer (Cr) | Sputtered Chrome (PVD). | Generally inert/passive. | High resistance (expensive). |
| Base Coat | Adhesion to glass surface. | Protected by layers above. | Delamination if alkali reaches it. |
| Glass Substrate | Structural integrity. | Etching (if pH > 10). | Loss of adhesion at interface. |
Understanding that the "metal" is sandwiched between polymers helps us realize that the topcoat is actually the primary line of defense, not the metal itself.
Which Electroplating Finishes on Glass Bottles Are Most Vulnerable to Alkali Attack?
Not all metallic shines are created equal; specifying the wrong metal or application method for an alkaline product guarantees failure. You must distinguish between vulnerable aluminum metallization and robust sputtered finishes.
Standard Vacuum Metallization (using Aluminum) is the most vulnerable to alkali attack, followed by Copper finishes; conversely, PVD Sputtering using Chrome, Stainless Steel, or Titanium targets offers significantly superior resistance.

The Weakest Link: Aluminum (Standard "Chrome" Look)
The vast majority of metallic glass bottles in the cosmetic and beverage market are Aluminum Vacuum Metallized. It is cost-effective and bright. However, it is chemically the most fragile.
Aluminum has almost zero resistance to caustic sodas or high-pH detergents. In our testing labs, a drop of 5% NaOH solution on exposed metallized aluminum will eat through to the glass in seconds, leaving a clear spot. If you are bottling a product with pH > 8, you are playing with fire unless the topcoat is impeccable.
Copper and Bronze Effects
Copper finishes are often achieved by tinting the topcoat over aluminum (safe if topcoat holds) or by sputtering actual copper (rare). Real copper is highly reactive to oxidation 5 and ammonia-based alkalis.
If the product contains ammonia (common in some cleaning agents) or amines, it will complex with the copper, turning it blue/green and dissolving it. This is a specific chemical vulnerability that differs from the brute-force dissolution of aluminum.
The Robust Alternatives: PVD Sputtering (Chrome/Nickel/Ti)
For clients needing extreme durability, we move to Magnetron Sputtering (PVD). Instead of vaporizing aluminum wire, we bombard a solid target of Chromium, Stainless Steel, or Titanium.
- Chromium: Extremely resistant to alkalis. It forms a passive oxide layer that stops further reaction.
- Nickel: Good resistance, though some concern exists regarding skin sensitization in cosmetics.
- Titanium: virtually inert.
The trade-off is cost and brightness. Sputtered chrome is darker (more "steely") than the bright white-silver of aluminum, and the process is 3-5x more expensive. But for a hair relaxer (pH 12) or a heavy degreaser, it is the only viable metallic option.
Vulnerability Hierarchy
| Finish Type | Metal Used | Alkali Vulnerability | Mechanism of Failure |
|---|---|---|---|
| Standard Metallization | Aluminum (Al) | Extreme | Rapid dissolution + Gas bubbling. |
| Real Copper PVD | Copper (Cu) | High | Oxidation & Amine complexation. |
| Spray Chrome | Silver Nitrate (Ag) | Moderate | Tarnish/Blackening (Sulfur/Alkali sensitivity). |
| Stainless PVD | Steel (Fe/Cr/Ni) | Low | Passive surface; very stable. |
| Chrome Sputtering | Chromium (Cr) | Very Low | Excellent chemical inertness. |
Choosing the "look" is easy; choosing the metal that survives your formula requires chemistry.
What Alkaline Conditions Typically Cause Electroplated Coating Discoloration, Peeling, or Pitting?
Underestimating the combined effects of pH, temperature, and time can lead to catastrophic leaching and aesthetic ruin. You need to define the exact environmental stress your bottle will face.
Discoloration and peeling typically occur when the pH exceeds 9.0, especially when combined with temperatures above 40°C or prolonged contact time (soaking), which accelerates the hydrolysis of the protective topcoat.
The pH Threshold: The Danger Zone
In my experience, the "Safe Zone" for standard aluminized bottles ends at pH 8.0.
- pH 8 – 10: This is the grey area. Products like mild soaps or shampoos. Here, the coating might survive if the exposure is brief (splash and wipe), but prolonged contact will cause the topcoat to haze or "blush" (absorb water).
- pH > 10: This is the destructive zone. Drain openers, hair perm solutions, industrial degreasers. At this level, the hydroxide ions ($OH^-$) aggressively attack the ester bonds in standard acrylic topcoats 6. Once the polymer chains are cut, the coating opens up, and the alkali destroys the metal underneath.
Temperature: The Accelerator
Temperature acts as a catalyst. A bottle might pass a resistance test at 20°C (Room Temp) but fail miserably at 45°C. In the supply chain, shipping containers can easily reach 50°C.
At elevated temperatures, the topcoat expands, increasing its permeability 7. Simultaneously, the chemical reaction rate between the alkali and the metal doubles for every 10°C rise. I have seen "stable" bottles arrive at a client’s warehouse in Dubai with the plating peeling off simply because the heat inside the container accelerated a minor incompatibility.
Contact Time and "Creep"
It’s not just about the liquid inside. The danger often comes from the environment.
- Spillage: A drip of product running down the neck. If not wiped, it sits there, concentrating as water evaporates.
- Cap Wicking: Capillary action draws liquid up between the threads and the coating. This is where peeling usually starts—at the neck finish.
- High Humidity: Alkaline environments aren’t always liquids. Ammonia fumes or high-salt environments near the ocean can corrode coatings over months.
Condition Impact Matrix
| Parameter | "Safe" Limit | "Risk" Limit | Consequence of Exceeding |
|---|---|---|---|
| pH Level | < 8.0 | > 9.5 | Topcoat hydrolysis; Metal etching. |
| Temperature | < 30°C | > 50°C | Coating expansion; Rapid reaction. |
| Contact Time | < 1 Hour (Wipe) | > 24 Hours | Saturation and penetration. |
| Concentration | Dilute (<1%) | Conc. (>5%) | Immediate chemical burn. |
| Detergent Type | Neutral | Caustic/Ammonia | Solvation of organic binders. |
If your product is a pH 11 hair cream, standard vacuum metallization is not just risky; it is unsuitable.
What Chemical Resistance and Adhesion Tests Should Buyers Require?
Visual approval of a "Golden Sample" is dangerous; you need data that proves the coating can survive the chemical reality of your product. Strict testing protocols are your only insurance against mass returns.
Buyers must require the Alkali Resistance Spot Test (NaOH), the Immersion Test (in bulk product), and the Cross-Hatch Adhesion Test (ASTM D3359) performed both before and after chemical exposure.

The Definitive Alkali Spot Test
To specifically test for the vulnerability we discussed, you cannot rely on general "chemical resistance." You need the NaOH Spot Test.
- Method: A 5% solution of Sodium Hydroxide (NaOH) is dropped onto the surface.
- Requirement: The drop must sit for a specified time (e.g., 1 hour or 24 hours depending on spec) covered by a watch glass.
- Pass Criteria: Zero discoloration, zero lifting. If the spot turns black or clear (metal gone), the batch is rejected.
Product Immersion: The Reality Check
Standard chemicals are good benchmarks, but your specific formula might have unique surfactants that penetrate coatings faster than pure NaOH.
- Method: Submerge the metallized bottle in your actual product at 45°C for 48 hours.
- Why: This tests the synergy of pH, chemical solvents (fragrance/alcohol), and temperature.
- Pass Criteria: No blistering at the interface. The coating should maintain its original gloss.
Dishwasher Resistance (For Reusables)
If the bottle is meant for the home (like a soap dispenser), it faces the dishwasher—the ultimate alkali torture chamber. Dishwasher detergents are highly alkaline (pH 10-12) and hot (60°C+).
- Standard: BS EN 12875-1 8.
- Cycle: 125 cycles.
- Reality: Most vacuum metallized bottles will not survive this. If you need this, you must specify it upfront so we can use specialized PVD or heavy-duty epoxy sealers.
The "Tape Test" (Post-Exposure)
A coating might look fine after soaking, but the bond to the glass might be destroyed.
- Method: ASTM D3359 9 (Cross-Hatch).
- Critical Step: Perform this test immediately after the chemical immersion or spot test.
- Pass Criteria: Class 5B. If the tape pulls the metal off only where the chemical was, the chemical resistance failed.
Buyer’s Test Protocol Summary
| Test Name | Target Agent | Methodology | Acceptance Standard |
|---|---|---|---|
| Alkali Spot | 5% NaOH | 24 hrs @ Room Temp | No discoloration/etching. |
| Bulk Immersion | Your Product | 48 hrs @ 45°C | No blistering/swelling. |
| Cross-Hatch | Adhesion | ASTM D3359 (Dry & Wet) | Class 5B (0% Loss). |
| Pencil Hardness | Abrasion | ASTM D3363 10 | > 2H (Ensures topcoat density). |
| Sweat Test | Synthetic Sweat | ISO 105-E04 | No tarnish (for handling). |
Never waive the immersion test using your own bulk liquid. It is the single most important validation step.
How Can Suppliers Improve Alkali Resistance for Electroplated Glass Bottles?
Standard off-the-shelf metallization is often insufficient for high-pH products. You must engineer the layers—pretreatment, metal choice, and topcoat—to build a robust defense system.
Suppliers can improve resistance by upgrading to high-crosslink UV Epoxy topcoats, increasing topcoat thickness (>25 microns), using PVD Chromium targets instead of Aluminum, and applying Silane pretreatments to prevent under-film corrosion.
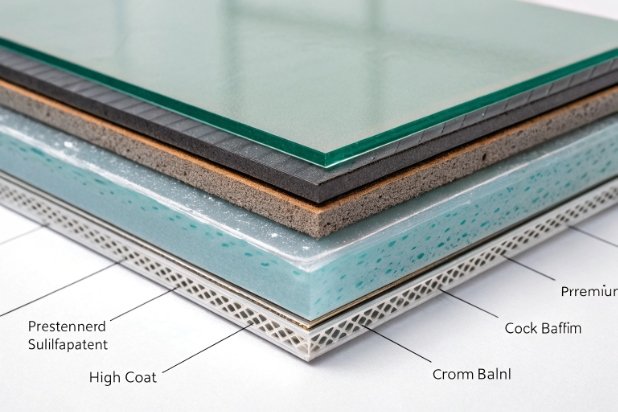
The Ultimate Shield: UV Epoxy Topcoats
The topcoat is the bodyguard. Standard acrylics are too porous for strong alkalis. At FuSenglass, when a client specifies alkali resistance, we switch to Dual-Cure UV Epoxies or High-Functionality Urethanes.
These resins have a much higher cross-link density. Imagine a fishing net: standard acrylic is a loose net that lets small fish (alkali ions) through. High-crosslink epoxy is a tight mesh that blocks them. This upgrade usually increases unit cost by 10-15%, but it is essential for safety.
Increasing Thickness (The Barrier Effect)
Sometimes, the solution is brute force. A standard topcoat might be 10-15 microns. By increasing this to 25-30 microns, we physically increase the distance the chemical must travel to reach the metal.
However, we must be careful—too thick, and the coating looks "plastic" and loses the sharp metallic reflection. We have to balance protection with aesthetics.
Material Substitution: Ditching Aluminum
As mentioned earlier, if the budget allows, stop using aluminum.
Sputtering Chromium or Stainless Steel eliminates the chemical reactivity at the source. Even if the topcoat fails, the metal underneath won’t dissolve or turn black. It might dull slightly, but it won’t peel. This is the "Nuclear Option" for durability.
Edge Sealing and Pretreatment
Failures often start at the edges (the bottom of the bottle or the neck).
- Silane Pretreatment: We apply a silane coupling agent to the glass before any coating. This chemically bonds the base coat to the glass, preventing "creep" where the alkali wiggles underneath the coating and lifts it off.
- Masking Strategy: For very high pH products, we often recommend masking the neck. We stop the metallization 10mm below the rim. This ensures that the product, when poured, never touches the metal edge, significantly reducing the risk of failure.
Improvement Strategy Checklist
| Upgrade Option | Mechanism | Cost Impact | Effectiveness (Alkali) |
|---|---|---|---|
| Epoxy Topcoat | Dense chemical barrier. | Moderate | High |
| Thicker Topcoat | Increased diffusion distance. | Low | Moderate |
| PVD Chromium | Inert metal layer. | High | Very High |
| Silane Pre-treat | Prevents under-film creep. | Low | High (Adhesion) |
| Neck Masking | Removes contact point. | Low | Absolute (for pouring) |
By implementing these engineering controls, we can transform a delicate decorative item into a durable functional package.
Conclusion
Electroplated finishes are beautiful but chemically fragile in alkaline environments. By understanding the vulnerability of aluminum, avoiding the pH "danger zone" (>9.0), and mandating PVD Chrome or Epoxy-sealed topcoats, you can safeguard your product’s luxury appeal against corrosion failure.
Footnotes
-
The process of using electric current to coat a conductive object with a thin layer of metal. ↩
-
A chemical property where a substance can react as either an acid or a base. ↩
-
A chemical reaction where a water molecule breaks one or more chemical bonds. ↩
-
An inorganic compound used in industrial applications, formed by dissolving aluminum in sodium hydroxide. ↩
-
A chemical reaction that involves the loss of electrons, often leading to rust or tarnish. ↩
-
A type of paint made of pigment suspended in an acrylic polymer emulsion. ↩
-
The rate of flow of a liquid or gas through a porous material. ↩
-
European standard for the resistance of domestic articles to mechanical dishwashing. ↩
-
Standard test methods for measuring adhesion by tape test. ↩
-
Standard test method for film hardness by pencil test. ↩








