When we think of salt spray tests, we usually think of metal corrosion. But if you are shipping glass bottles across the ocean or storing them in coastal warehouses, salt is a silent aggressor. Does this exposure weaken the bottle’s ability to handle heat?
No, salt spray itself does not chemically alter the bulk glass or change its Coefficient of Thermal Expansion. However, it severely degrades surface coatings, labels, and closures. This surface degradation significantly lowers the bottle’s "system" thermal resistance, leading to coating delamination, print peeling, and an increased risk of breakage due to stress corrosion.

The Surface is the Shield
At FuSenglass, we remind clients that a glass bottle is a system: the glass substrate, the chemical coating (Hot/Cold End), the decoration (ink/foil), and the closure. While the silica glass is chemically inert to salt, the shield protecting it is not.
"Dive Deeper" into the chemistry: Salt spray (Sodium Chloride mist) creates a highly corrosive electrolytic environment.
-
Glass Strength: Glass strength is surface-dependent. Salt accelerates "Stress Corrosion." If there is a microscopic crack (Griffith flaw 1) on the glass surface, the presence of water and ions lowers the energy needed for that crack to grow.
-
Thermal Impact: When you take a salt-exposed bottle and subject it to thermal shock 2 (e.g., hot filling), the weakened surface coatings fail to protect the glass from abrasion, and the pre-corroded micro-cracks propagate faster. The glass didn’t change, but its defenses were stripped away.
Primary Impact Areas
Salt spray attacks the interface between materials.
-
Glass-to-Coating: It undercuts the bond.
-
Ink-to-Glass: It dissolves the adhesion promoters.
-
Metal-to-Glass: It corrodes foil stamps.
Does salt spray exposure reduce thermal shock resistance?
It is a common misconception that salt "eats" the glass. It doesn’t. It eats the strength of the glass.
Salt spray exposure does not change the glass’s melting point or expansion rate, but it promotes "Stress Corrosion Cracking" at the surface. By deepening existing micro-flaws and stripping away protective lubricants, it makes the bottle more brittle and susceptible to failure during subsequent thermal shock events.

The Mechanism of Stress Corrosion
Water alone weakens glass (hydrolytic attack). Salt water is worse.
-
The Reaction: The sodium ions in the salt solution can exchange with ions on the glass surface, creating a localized stress field.
-
The Crack Tip: At the tip of a tiny scratch, the corrosive solution breaks the Silicon-Oxygen bonds ($Si-O-Si$) faster than dry air.
-
The Result: A scratch that would have been stable at 80°C might now propagate at 60°C because the chemical resistance at the crack tip has been compromised.
Breakage Risk vs. Thermal Performance
-
Pure Thermal Performance: The ability of the glass to conduct heat or expand remains unchanged.
-
Breakage Risk: Drastically increases. A salt-weathered bottle is a weaker vessel. It will fail a thermal shock test (ASTM C149 3) at a lower temperature differential ($\Delta$T) than a pristine bottle.
Which parts are most impacted by salt spray and heat?
The glass usually survives; the decoration usually dies. The "premium" look you paid for is the first casualty.
Metallic finishes (foiling, metallization) and organic coatings (UV printing, spraying) are the primary victims. Salt spray penetrates micropores in these layers, causing oxidation and blistering, which is then exacerbated by thermal expansion during heating, leading to total delamination.

1. Vacuum Metallization & Hot Stamping
This is the most vulnerable category.
-
The Attack: These are thin layers of aluminum or metal deposited on the glass. Salt creates a galvanic cell. The metal oxidizes (rusts/tarnishes).
-
The Heat: When heated, the metal expands. Since the corrosion has already loosened its grip on the glass, the foil flakes off or "spiders" (webs of cracks).
2. Closures (Caps)
-
Aluminum/Tinplate Caps: Highly susceptible to white rust or red rust.
-
The Seal: Corrosion can creep under the liner (plastisol/PE), compromising the hermetic seal. When the bottle is heated (pasteurization 4), the compromised seal blows out or leaks.
3. Organic Inks (Screen Print/Spray)
-
The Attack: Salt water is a solvent. It can swell the polymer matrix of the ink.
-
The Heat: Swollen ink loses adhesion. Thermal expansion shears it off. You get "blistering" or "orange peel" effects.
Vulnerability Matrix
| Component | Salt Sensitivity | Thermal Consequence | Visual Failure Mode |
|---|---|---|---|
| Bare Glass | Very Low | Increased crack propagation. | None visible (until breakage). |
| Hot/Cold End Coating | Moderate | Loss of lubricity. | Scuffing -> Breakage. |
| Organic Spray Color | High | Loss of adhesion. | Peeling / Blistering. |
| Gold/Silver Foil | Extreme | Oxidation + Flaking. | Black spots / "Spidering". |
| Metal Cap | High | Thread corrosion. | Leaking / Rust stains. |
Can salt residue worsen heat-cycle failures?
If you don’t wash the salt off, it keeps working.
Yes, salt residue is hygroscopic 5 (attracts water). Even in a dry warehouse, salt crystals on the surface pull moisture from the air, creating a concentrated corrosive brine that continues to undercut coatings and inks. During heating, these crystals can also create localized "hot spots" or abrasive points that trigger coating cracks.

The Hygroscopic Cycle
-
Spray: Bottle gets salt misted.
-
Dry: Water evaporates, leaving salt crystals.
-
Humid Day: Crystals suck moisture, re-liquefy, and resume corrosion.
-
Heating Event: The water boils off rapidly under the coating, causing Steam Blistering. The coating pops off.
"Creep" Corrosion
Corrosion products (rust/oxide) take up more volume than the metal itself.
-
As the metal decoration corrodes, it expands.
-
This expansion pushes the ink/foil away from the glass.
-
Add thermal expansion to this, and the decoration essentially pushes itself off the bottle.
How should B2B buyers set a combined test plan?
Don’t test them separately. Real life happens in sequence. Shipping (Salt) -> Warehousing (Heat/Cold) -> Filling (Heat).
Buyers typically follow a sequential protocol: 24-48 hours of Salt Spray (ASTM B117), followed by rinse/dry, and then immediately subjecting the samples to Adhesion Testing (Tape Test) and Thermal Cycling. This mimics the real-world supply chain and reveals hidden weaknesses.
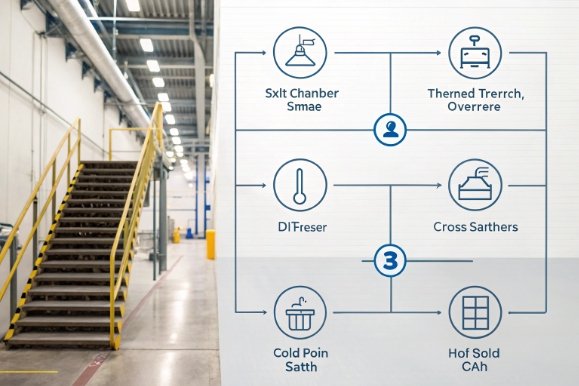
The "Torture Test" Protocol
At FuSenglass, we recommend this sequence for premium decorated ware.
Step 1: The Salt Chamber (ASTM B117)
-
Duration: 24 Hours (Standard) or 48 Hours (Marine transit simulation).
-
Condition: 5% NaCl fog at 35°C.
-
Pass Criteria: No visible corrosion, pitting, or discoloration at 1x magnification.
Step 2: The Rinse & Rest
-
Rinse with deionized water (remove bulk salt).
-
Air dry for 2 hours. (Do NOT bone dry; let the moisture do its work).
Step 3: Thermal Cycling (The Trigger)
-
Cycle: Place in oven at 60°C (1 hour) -> Freezer at -10°C (1 hour). Repeat 3 times.
-
Purpose: The expansion/contraction will try to dislodge any coating loosened by the salt.
Step 4: The Evaluation
-
Cross-Hatch Tape Test (ASTM D3359): Cut the grid, pull the tape.
-
Pass Criteria: Grade 4B or 5B (0-5% removal). If the ink comes off here, it survived salt but died from thermal stress induced by salt.
Test Plan Summary Table
| Test Phase | Condition | What it Reveals | Passing Standard |
|---|---|---|---|
| 1. Salt Spray | ASTM B117 6 (24h) | Chemical resistance of ink/metal. | No visual rust/tarnish. |
| 2. Thermal Cycle | -10°C to 60°C (3x) | Adhesion under stress. | No blistering/cracking. |
| 3. Tape Test | ASTM D3359 7 | Bond strength after abuse. | 5B (Perfect Adhesion). |
| 4. Thermal Shock | ASTM C149 | Glass structural integrity. | Survive $\Delta$T 42°C. |
Conclusion
Salt spray doesn’t melt glass, but it dismantles the armor. By stripping coatings and corroding decorations, it leaves the bottle naked and vulnerable to thermal stress. A combined testing protocol is the only way to ensure your packaging survives the journey from the ocean container to the customer’s hands intact.
Footnotes
-
A theoretical micro-crack in brittle materials that acts as a starting point for fracture. ↩
-
Failure caused by sudden temperature changes creating expansion/contraction stress. ↩
-
Standard test method for determining the thermal shock resistance of glass containers. ↩
-
A sterilization process using heat, which puts thermal and pressure stress on containers. ↩
-
A property of a substance to absorb moisture from the air, like salt. ↩
-
Standard practice for operating salt spray (fog) apparatus for corrosion testing. ↩
-
Standard test methods for measuring adhesion by tape test. ↩








